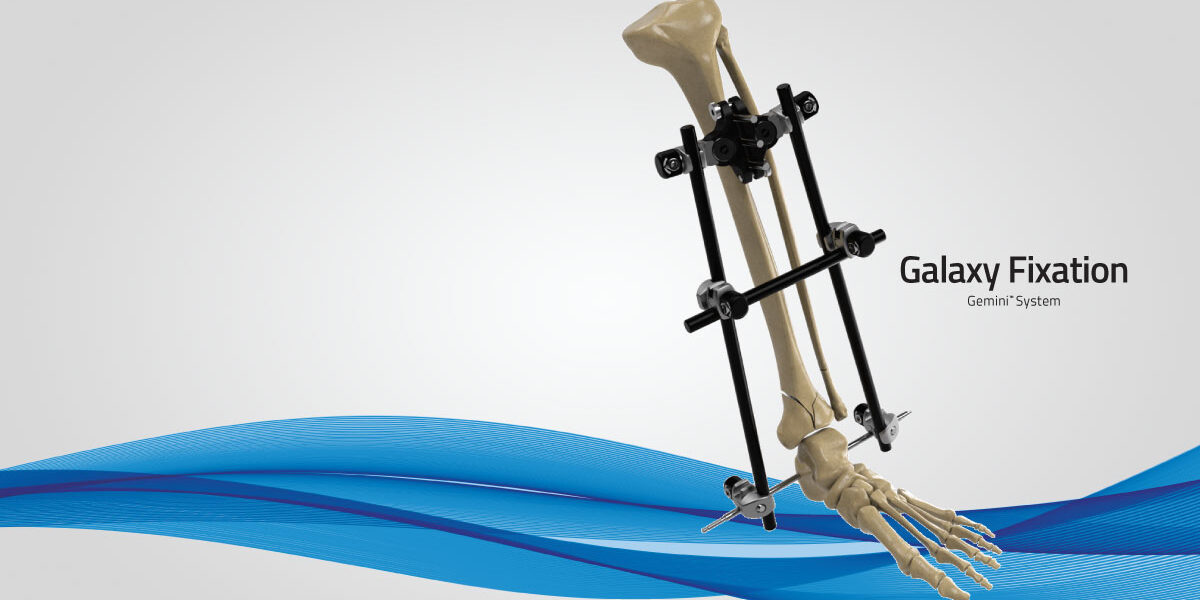
Orthofix Announces First Patient Procedures and Full Commercial Launch of the Galaxy Fixation Gemini System and Expanded Sterile Kit Offerings for Orthopedic Trauma Procedures
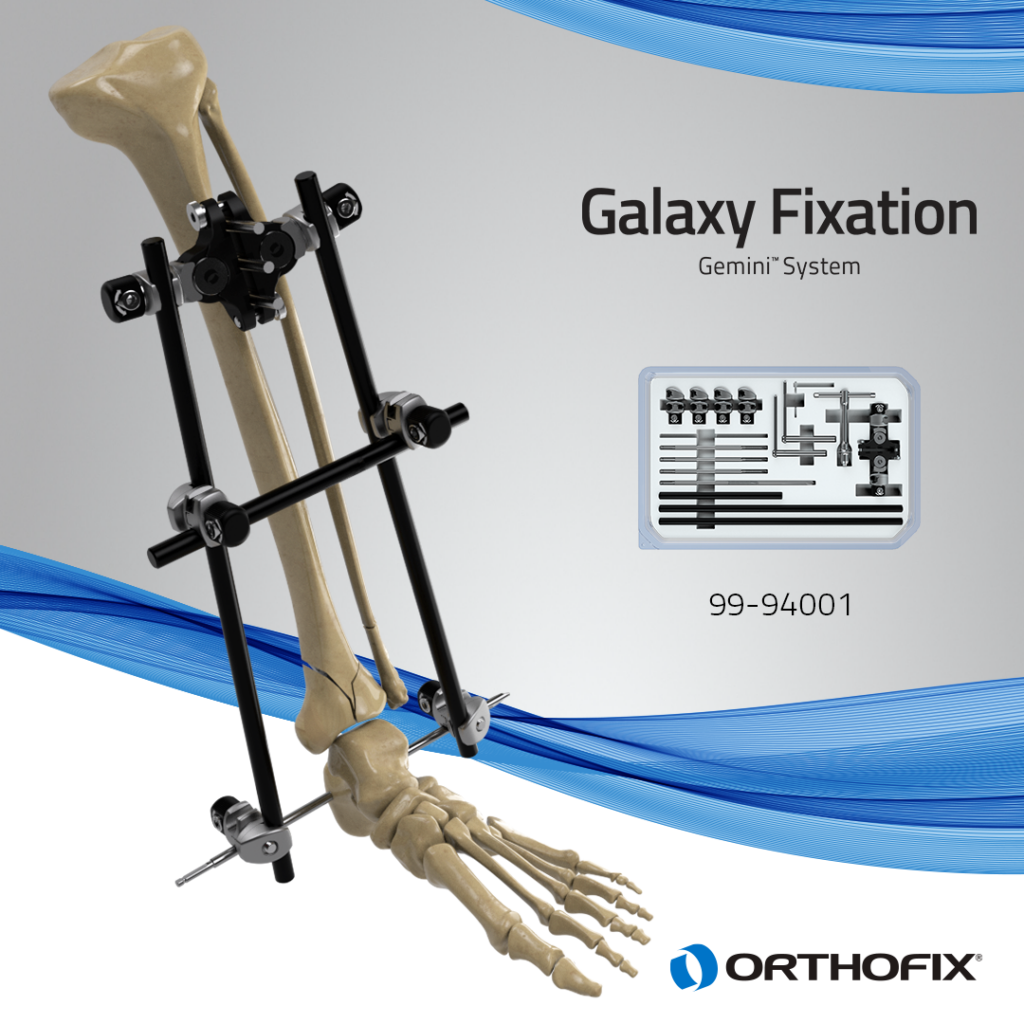
LEWISVILLE, TEXAS — September 20, 2023 — Orthofix Medical Inc. (NASDAQ:OFIX), a leading global spine and orthopedics company, today announced the full U.S. commercial launch and successful completion of the first cases with the Galaxy Fixation Gemini™ system. The latest in the Galaxy product line, this stable external fixation system is provided in several sterile procedure kit configurations as a quick off-the-shelf solution for treating fractures that result from trauma in the lower and upper limbs.
“In trauma settings, it is critical that we respond quickly to manage the damage to the impacted limb,” said Dr. Evengy Dyskin, Clinical Assistant Professor of Orthopaedics, University of Buffalo, in Buffalo, NY. “The all-inclusive nature of the sterile kits eliminates spatial constraints, enabling application in a trauma bay or intensive care unit setting, which would not be possible with a traditional pin-to-bar system.”
Sterile kits eliminate the need for sterilization of trays which takes an average of 139 minutes1 to process and adds cost to healthcare facilities. Sterilization of trays also results in increased operating room (OR) delays and costs due to packaging, restocking and tray contamination. Average cost to a facility for OR delays related to tray contamination is $1,0811 which can be avoided through the use of prepacked sterile kits.
In particular, the Galaxy Fixation Gemini ankle kit is the only pin-to-bar system with specific clamps available in a sterile kit configuration giving surgeons more efficient solutions in lower extremity trauma situations where time is an important factor. The ankle kit, developed specifically to meet the needs of U.S. surgeons, includes a double multiscrew clamp to facilitate rapid insertion of tibial half-pins and is complemented by the foot support and first metatarsal sterile kits when a more robust construct is desired. It is estimated that ankle fractures occur in 187 per 100,000 persons per year in the U.S.2
“We are excited to expand our external fixation offerings in the U.S. for trauma through the introduction of the Galaxy Fixation Gemini System in several sterile packed configurations,” said Kim Elting, President of Global Orthopedics. “This streamlined pin-to-bar configuration is the latest example of our leadership in sterile kit solutions, which improve OR efficiencies and meaningfully decrease costs for the hospital and also for our business.”
Orthofix further expanded its sterile kit offerings with the global launch of the CalcFix Plus Calcaneal Minifixator™ System earlier this year. The CalcFix Plus represents the latest version of the original CalcFix Fixator device designed to treat calcaneal fractures using a more minimally invasive external fixation approach compared with internal fixation and includes updates to the design that reduce the number of operative steps to apply.

The Galaxy Fixation Gemini and the CalcFix Plus Systems add to Orthofix’s extensive portfolio of over 50 sterile kit procedural solutions available globally and demonstrate Orthofix’s continued investment in providing off-the-shelf solutions to meet the needs in the acute trauma, military and humanitarian crisis settings.
Those attending the AOFAS Annual Meeting in Louisville, KY Sept. 20-22 can learn more about the Galaxy Fixation Gemini System and the CalcFix Plus Calcaneal Minifixator System by visiting booth 613.
1 Ly JA, Wang WL, et al. Arch Bone Jt Surg, 2022 May; 10(5): 420-425.
2 Source (SmartTrack, 2023).