CervicalStim
Spinal Fusion Therapy
The CervicalStim™ device is the only bone growth stimulation therapy approved by the FDA as a noninvasive, adjunctive treatment option for cervical fusion in patients at high-risk for non-fusion. The device uses a pulsed electromagnetic field (PEMF) to induce a low-level electrical field at the fusion site which stimulates bone healing at a molecular, cellular, and tissue level. With an overall clinical success rate of 84 percent, the CervicalStim device increases fusion success significantly by 22 percent when used adjunctively to surgery.1,2
The CervicalStim device has been approved by the FDA to be worn after cervical spine fusion surgery in patients at risk for non-fusion.1,2 For complete prescribing information, please refer to the Instruction Manual.
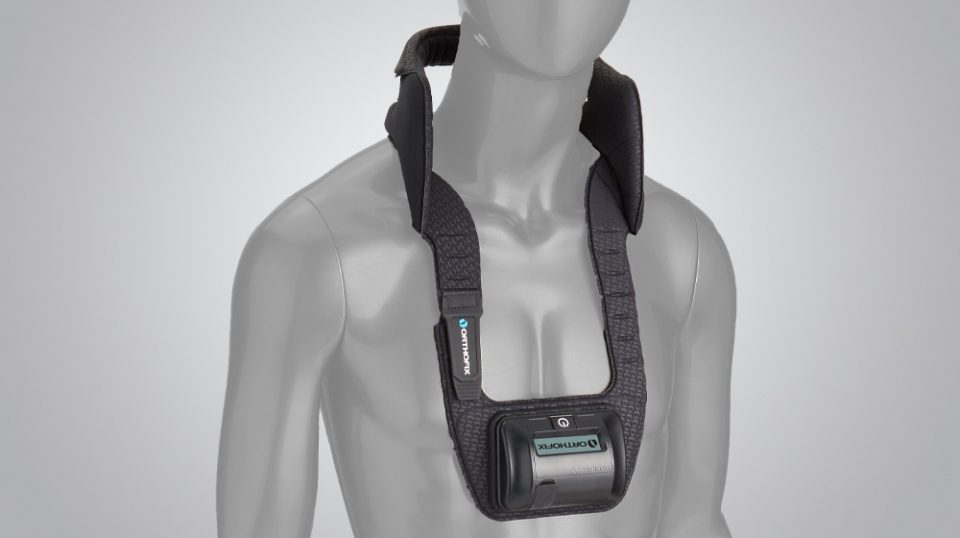
It is a single-piece device that is lightweight, flexible and portable, allowing freedom of movement during treatment. An LCD and audible alarm provide important feedback during treatment such as the operational status, treatment time remaining, battery capacity, etc.
Designed for patient ease of use
- Works effectively when worn over clothing or bracing
- Single-piece, cordless design allows for ease of placement and patient mobility
- The STIM onTrack™ app is patient-friendly and provides patients with a treatment calendar, therapy reminders, and additional educational resources.*
This device utilizes Orthofix PEMF technology that provides 360 degrees of treatment coverage around the fusion site, covering up to five vertebral levels.3
Why do physicians prescribe a CervicalStim device?
- High clinical success rates1,2
- Statistically significant results for patients who smoke or have a multi-level fusion1,2
- PEMF signal covers 360 degrees around the fusion site3
- Coverage up to 5 vertebral levels3
- Supported by the North American Spine Society’s coverage recommendations4
- The STIM onTrack app enables physicians to remotely view patient adherence to prescribed treatments when paired with the DIRECT Physician Portal
To understand the reasons behind physicians recommending the use of the CervicalStim device, please explore our interactive demonstration.
*The STIM onTrack app is available as an accessory for US model devices only
Patient Education Websites
Support Material
Footnotes
- PMA P030034. December 2004.
- Foley KT, Mroz TE, Arnold PM, et al. Randomized, prospective and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J. 2008;8(3):436-442. Full article available at PubMed.gov
- Data on file. Field mapping analysis conducted by M. Zborowski, Ph.D., Cleveland Clinic.
- spine.org/PolicyPractice/CoverageRecommendations/AboutCoverageRecommendations.aspx
*Money back guarantee does not apply to wholesale orders.


