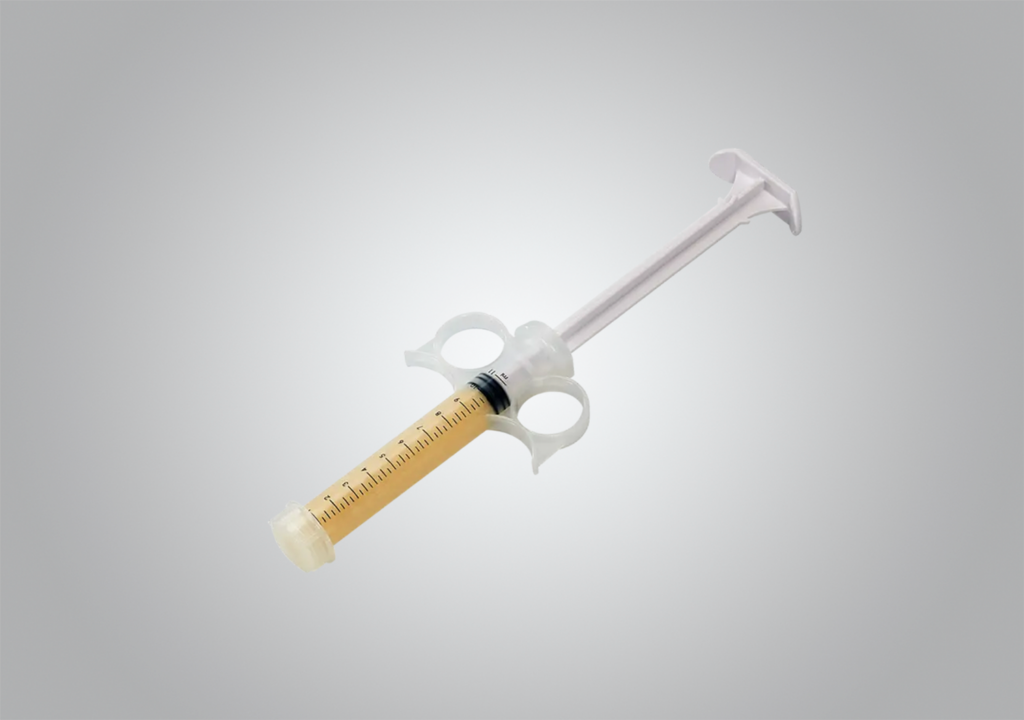
Accell Evo3c
Our Accell Evo3®c grafts combine demineralized bone matrix with patented Accell® Bone Matrix in a bioresorbable, reverse-phase medium carrier. Provided in an open-bore syringe, the putty is moldable, resists irrigation, and includes cancellous chips. The putty may be implanted directly from the syringe.
Key Features
Accell Technology
The patented Accell process yields Accell Bone Matrix (ABM). ABM is an open-structured, dispersed form of DBM.
Superior handling
The unique, poloxamer, reverse phase medium carrier, which becomes more viscous at body temperature, provides exceptional handling and containment characteristics. The graft is moldable, packable, mixable and resists irrigation.
Verified osteoinductive potential
Every lot of DBM is tested in a validated, in vitro assay to verify osteoinductive potential.
Expert processing
Ground cortical bone and cancellous bone obtained from AATB-accredited tissue banks is processed into DBM in our state-of-the-art facility. The demineralization process is tightly controlled to consistently produce demineralized bone.
Electron beam sterilization
The processing of DBM and ABM is completed under aseptic conditions in our state-of-the-art facility. As the last step in manufacturing, the graft is sterilized via electron beam irradiation.
Cancellous bone
Cancellous bone supplies an osteoconductive scaffold.
Technologies