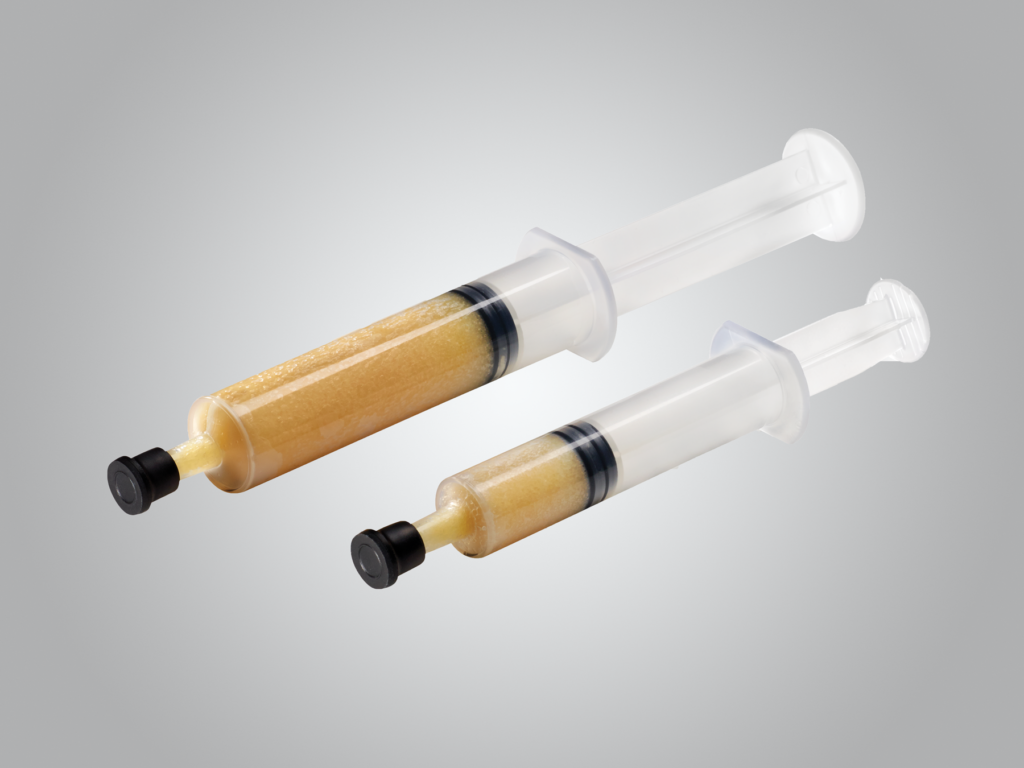
Accell Connexus
Our Accell Connexus® grafts combine demineralized bone matrix with patented Accell® Bone Matrix in a bioresorbable, reverse phase medium carrier. Provided in a syringe, the putty is moldable and resists irrigation. The putty may be implanted directly from the syringe.
Key Features
- Accell Technology – The patented Accell process yields Accell bone matrix (ABM). ABM is an open-structured, dispersed form of DBM
- Superior Handling – The unique, poloxamer, reverse phase medium carrier, which becomes more viscous at body temperature, provides exceptional handling and containment characteristics. The graft is moldable, packable, mixable and resists irrigation
- Verified Osteoinductive Potential – Every lot of DBM is tested in a validated, in vitro assay to verify osteoinductive potential
- Expert Processing – Ground cortical bone and cancellous bone obtained from AATB-accredited tissue banks is processed into DBM in our state-of-the-art facility. The demineralization process is tightly controlled to consistently produce demineralized bone
- Electron Beam Sterilization – The processing of DBM and ABM is completed under aseptic conditions in our state-of-the-art facility. As the last step in manufacturing, the graft is sterilized via electron beam irradiation
Technologies