Accell Connexus
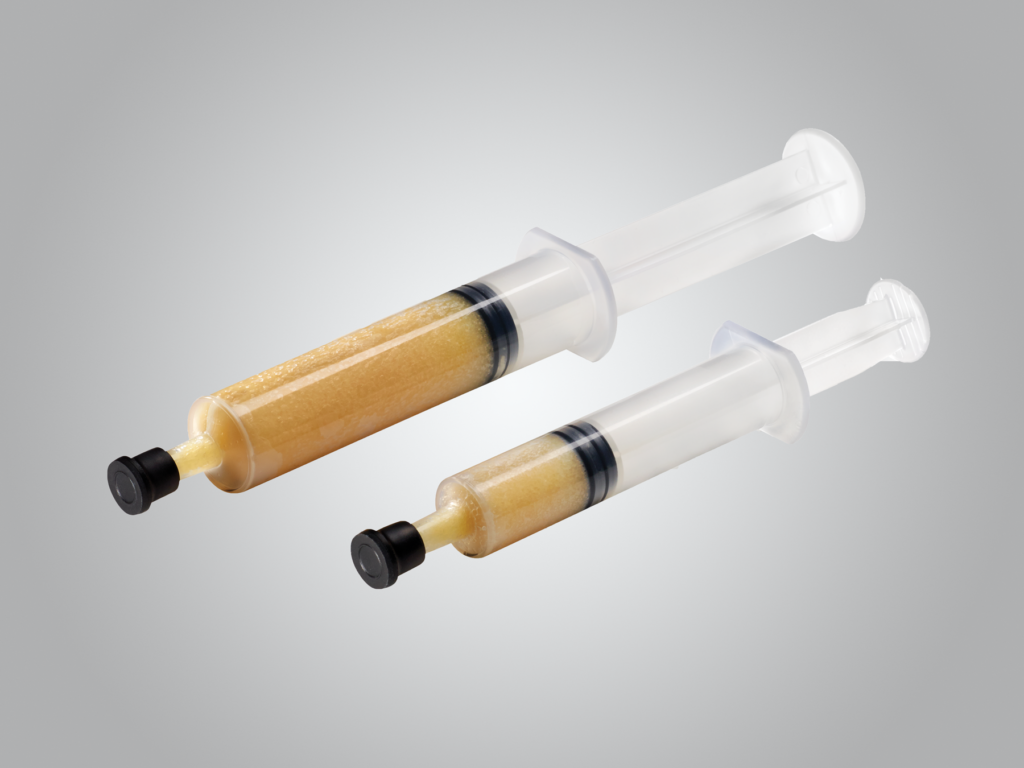
DBM Putty with Accell™ Bone Matrix
Accell Connexus™ combines patented Accell™ Bone Matrix (ABM) and particulate DBM to provide a biphasic release of growth factors while the reverse phase medium (RPM) carrier contributes to the exceptional handling characteristics and irrigation resistance of Accell Connexus.1
Key Features
- Contains Accell Bone Matrix (ABM)
- RPM carrier creates a moldable and irrigation-resistant putty
- Compatible with O-Genesis™ and RAPID™ graft delivery systems
- Osteoinductive potential is tested on every lot
Technologies
Footnotes
- Data on file.