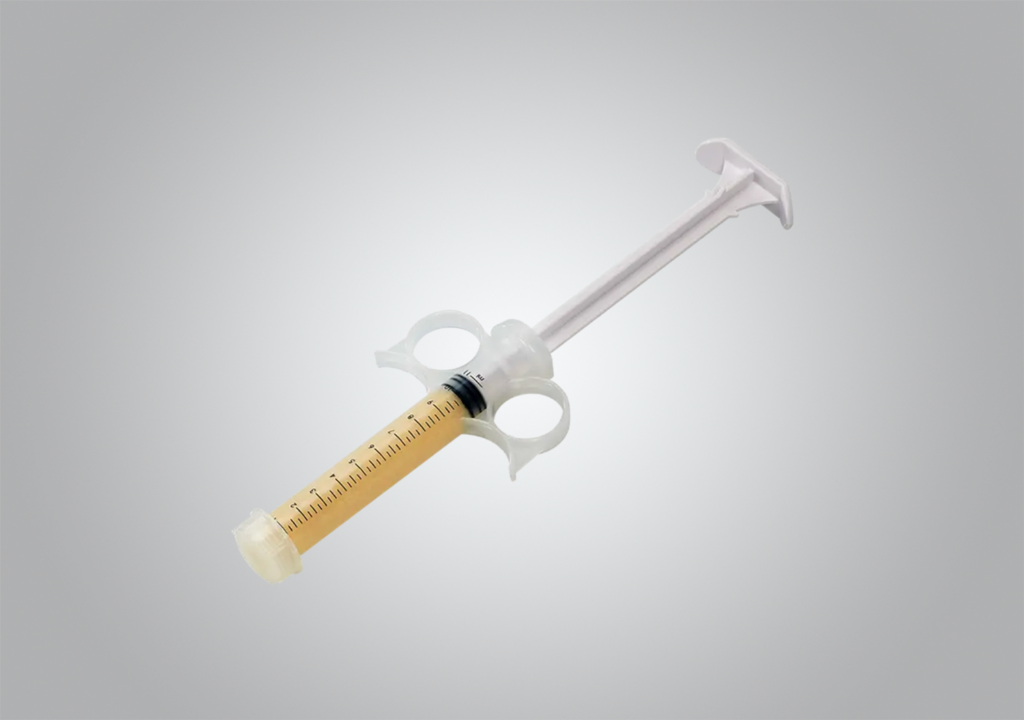
OsteoSurge 300
Our OsteoSurge® 300 grafts combine demineralized bone matrix with patented Accell® Bone Matrix and cancellous bone in a bio-resorbable, reverse-phase medium carrier. Provided in an open-bore syringe, the putty is moldable, resists irrigation and includes cancellous chips.
Key Features
Accell
- The patented Accell process yields Accell bone matrix (ABM). ABM is an open-structured, dispersed form of DBM. OsteoSurge 300 and OsteoSurge 300c DBM contain the highest concentration of ABM available in our products.
Superior Handling
- The unique, poloxamer, reverse-phase medium carrier, which becomes more viscous at body temperature, provides exceptional handling and containment characteristics. The graft is moldable, packable, mixable, and resists irrigation.
Verified Osteoinductive Potential
- Every lot of DBM is tested in a validated, in vitro assay to verify osteoinductive potential.
Expert Processing
- Cortical bone and cancellous bone obtained from AATB-accredited tissue banks is processed into DBM in our state-of-the-art facility. The demineralization process is tightly controlled to consistently produce demineralized bone.
Electron Beam Sterilization
- The processing of DBM and ABM is completed under aseptic conditions in our state-of-the-art facility. As the last step in manufacturing, the graft is sterilized via electron beam irradiation.
Technologies